Ep. 180: All About Semaglutide, Ozempic, Wegovy and Rybelsus
Listen, Rate & Subscribe*
Apple Podcasts // Spotify // Google Podcasts // Stitcher // Amazon Music // YouTube
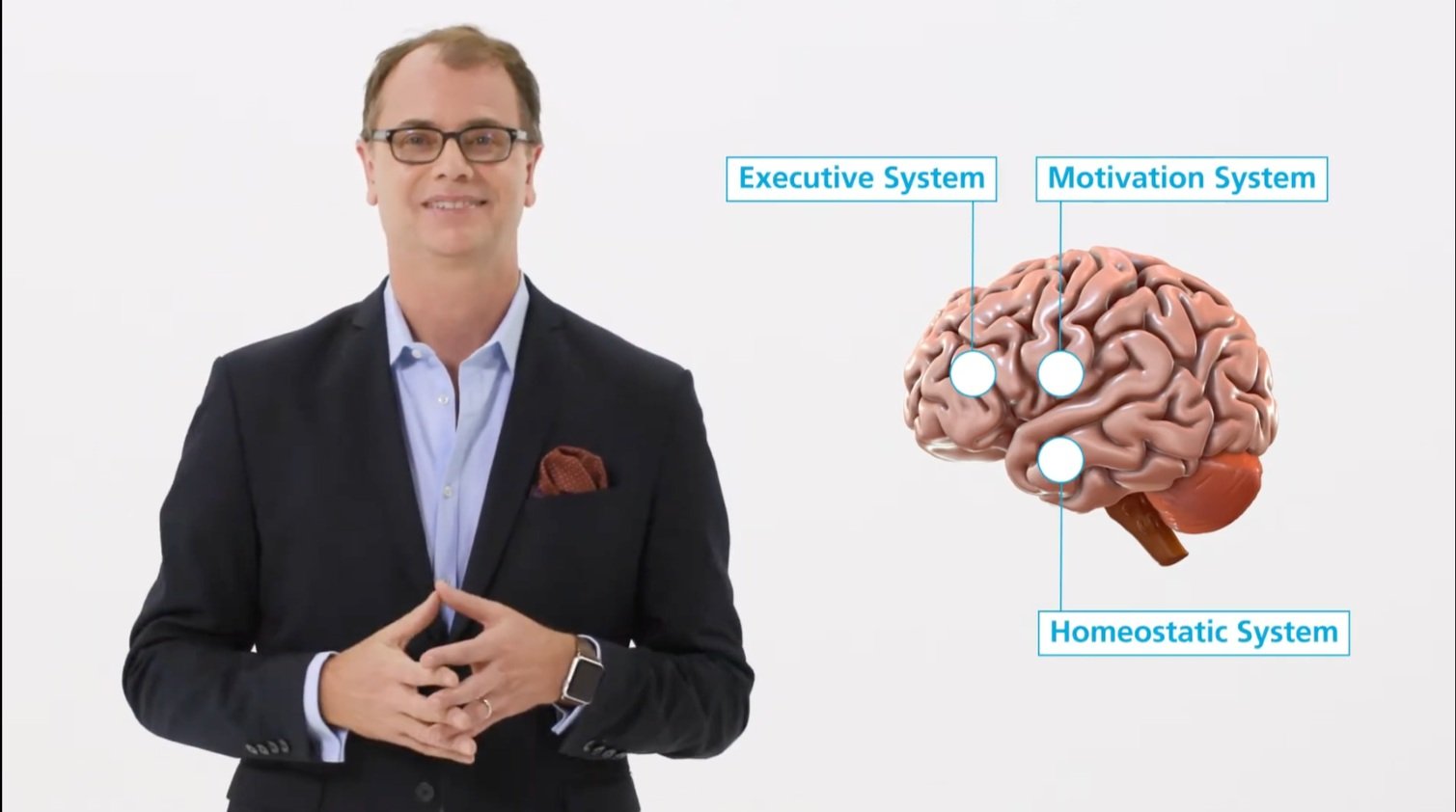
Medcan’s director of weight management, Dr. David Macklin, in his presentation on obesity and the brain.
Last year, Medcan director of weight management Dr. David Macklin predicted the diabetes medication semaglutide would be a game changer for those seeking help to lose weight. Now known by trade names like Ozempic, Wegovy and Rybelsus, today semaglutide is advertised on billboards and in TV commercials — and the hashtag has more than 330 million views on Tiktok. So we invited back Dr. Macklin to get his perspective on the medication, now that it’s actually on the market and being used by hundreds of thousands of people across North America. Joined by Medcan CMO Dr. Peter Nord, Dr. Macklin explains semaglutide’s benefits, side effects and costs — and describes what’s different about Medcan’s approach to weight management in the semaglutide era.
DISCLOSURE
In addition to his role at Medcan, Dr. Macklin helped to write the Canadian clinical guidelines for obesity. He also receives consulting fees, honoraria, and licensing fees from Novo Nordisk. Novo Nordisk is the manufacturer of semaglutide (trade name Ozempic).
LINKS
Book an appointment or read more about Medcan’s Weight Management program.
Check out Dr. David Macklin’s Twitter page.
Read the Canadian Adult Obesity Clinical Practice Guidelines, co-authored by Dr. Macklin.
Listen to Dr. Macklin’s first Eat Move Think episode about semaglutide.
What Is Ozempic and Why Is It Getting So Much Attention? in the New York Times.
Read this article in CNN Health to track the sales of Ozempic since 2018.
Read After weight loss, Alzheimer's may be next frontier for drugs like Ozempic in Reuters.
Check out some scientific research on the effects of semaglutide:
Read about the results of the SUSTAIN 6 trial: Cardiovascular Safety and Benefits of Semaglutide in Patients With Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6 in Frontiers in Endocrinology.
Once-Weekly Semaglutide in Adults with Overweight or Obesity in The New England Journal of Medicine 2021.
Semaglutide for weight loss in Canadian Family Physician 2021.
The New England Journal of Medicine on tirzepatide versus semaglutide for patients with Type 2 diabetes.
Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial published in The Lancet.
INSIGHTS
03:15 Dr. Macklin speaks on the culture of obesity treatment in Canada, and how it’s changing.
09:05 Hear a breakdown of the three pillars of obesity treatment. What determines someone’s success in a weight management program?
14:44 How exactly does semaglutide affect the body, the brain and appetite?
16:15 How long does semaglutide take to work, and what sort of results can people expect to see?
19:35 People are seeing outcomes beyond weight loss. What other benefits can come with taking semaglutide?
22:06 What side effects or risks come with taking semaglutide?
24:50 Unlike previous weight loss medications, semaglutide actually lowers your risk of cardiovascular disease and stroke.
29:07 What happens when you stop taking semaglutide?
32:07 How much does it cost to take semaglutide in Canada?
34:38 When can Canadians expect the pill form of semaglutide to be available?
37:37 There is exciting early research that suggests semaglutide might help to manage the onset of dementia.
40:01 What happens when someone who is at a normal weight tries semaglutide?
42:03 Listen to Dr. Macklin’s predictions for the next few years in the field of weight management.
*LEGAL
This podcast episode is intended to provide general information about health and wellness only and is not designed, or intended to constitute, or be used as a substitute for, medical advice, treatment or diagnosis. You should always talk to your Medcan health care provider for individual medical advice, diagnosis and treatment, including your specific health and wellness needs.
The podcast is based on the information available at the time of preparation and is only accurate and current as of that date. Source information and recommendations are subject to change based on scientific evidence as it evolves over time. Medcan is not responsible for future changes or updates to the information and recommendations, and assumes no obligation to update based on future developments.
Reference to, or mention of, specific treatments or therapies, does not constitute or imply a recommendation or endorsement. The links provided within the associated document are to assist the reader with the specific information highlighted. Any third-party links are not endorsed by Medcan.